Abstract
Introduction: As conditions like atrial fibrillation and venous thromboembolism (VTE) become more prevalent in the United States, the use of direct oral anticoagulants (DOACs) is increasing. Patients presenting to the hospital with indications of thrombosis are often started on unfractionated heparin (UFH) infusion, which has traditionally been monitored using laboratory based PTT and anti-Xa assays. While monitoring UFH with anti-Xa assays have been shown to correlate with better outcomes than dose adjustments guided by PTT, anti-Xa assays are susceptible to interferences from DOACs taken prior to admission and can result in inappropriate dose adjustments that can negatively impact patient care.
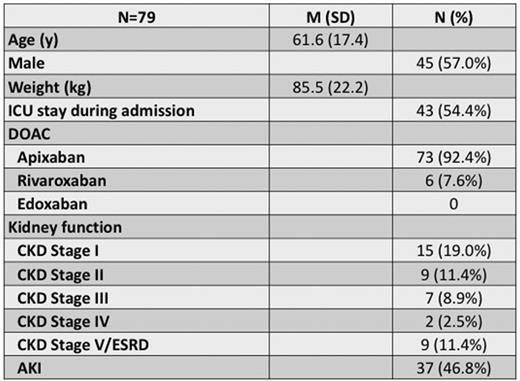
Methods: We derived a novel assay, termed corrected heparin (CH), using quantified values from a chromogenic anti-Xa assay with heparin calibrators prior to and following heparinase treatment to detect any residual DOAC still present within the patient sample. We retrospectively assessed 79 adult patients admitted to our institution who had their UFH infusion monitored using the CH assay due to known DOAC use. These patients were continually monitored by the CH assay and the PTT (unreported) if they had detectable DOAC levels (>0.1 IU/mL by anti-Xa). Of the patients included in the study, 92% were administered apixaban, while 8% were administered rivaroxaban prior to admission and all received UFH while admitted.
Results: To assess linearity of anti-Xa assay for this purpose, patient samples with both UFH and apixaban present were diluted with drug-free plasma and displayed good correlation (R2=0.999) throughout the linear range of the assay. Of the 79 patients, 53 patients reached the therapeutic range by the PTT and CH assays, 17 reached the therapeutic range by the CH assay only, 2 reached the therapeutic range by PTT only, and 2 never reached the therapeutic range with either assay. The average time to therapeutic dosing level for the CH assay was 9 hours after initiation of UFH infusion. Using the CH assay, we were able to determine how long the DOAC persisted and was detected by the anti-Xa assay. At 12 hours after the last DOAC dose, more than 80% of patients still had detectable DOAC levels capable of interfering with the anti-Xa assays. The majority of patients (80%) still had measurable anti-Xa assay interference from the DOAC until 72 hours after the last dose. We evaluated the role of kidney function as a predictor for prolonged interference in the anti-Xa assay however, clearance rates of apixaban were difficult to predict based on kidney function status alone.
Conclusions: Our CH assay has provided clinicians with an assay that accounts for the presence of residual DOAC and allows patients to reach therapeutic heparin levels faster. Our study also highlights the delayed clearance of commonly prescribed DOACs, which can be longer than expected and independent of kidney function status resulting in prolonged interference in anti-Xa assays that can lead to improper dose adjustments and potentially negative outcomes for patients on UFH infusion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal